New insights on the early stages of HIV infection in the human body
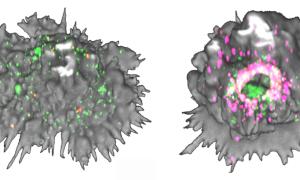
In an article published in eLife, researchers discover that nanoclusters of the protein Siglec-1, which form in the active dendritic cells of the immune system, are crucial to increase the capture of HIV-like particles
The human immunodeficiency virus (HIV) is a virus that attacks the body's immune system. If not treated, it can lead to the autoimmune deficiency syndrome (AIDS). As happens with other viruses, when a foreign substance enters our body, the immune system recognizes it as "non-self" and launches an immune response, which consists of various cells, tissues, and organs that work together to identify, attack and eliminate that foreign substance. In the case of the HIV virus, it first enters the body via the dendritic cells, the immune cells that are in contact with the external environment, patrolling our body in the search for pathogens and protecting us from infections.
The capture and transmission of the virus
The dendritic cells are the ones responsible for processing foreign proteins, molecules or particles, and presenting them to the immune system T-cells, acting as messengers and initiating the immune response.
A critical element that helps the dendritic cells recognize and bind to the virus is a group of membrane proteins that distinguish between self and non-self. One such protein, called Siglec-1, plays a key role in the early stages of HIV infection specifically in the capture and transmission of the virus.
When HIV enters the body, it first encounters the mucosal surfaces and binds to various molecules. Then, dendritic cells expressing Siglec-1 can capture and transmit the virus to other cells, initiating an immune response. But in this transportation journey, HIV-1 viruses can also use the dendritic cells as vehicles to infect the helper T-cells, also known as CD4+cells, spreading the infection further in a process known as trans-infection. This means that, although it can help to initiate the immune response, it can also facilitate the infection.
While previous studies, including those from IrsiCaixa, have identified Siglec-1 as the main receptor on activated dendritic cells that bind to specific molecules of the HIV-1 particles, the specific mechanisms of how this happens are still unknown. Understanding the role of Siglec-1 in the immune response to HIV is critical to developing effective treatments and therapies for people living with HIV/AIDS.
Studying the formation of nanoclusters and compartments
In a new article, a team of researchers describe the mechanisms underlying the capture of HIV-1 viruses in the dendritic cells, and the role that Siglec-1 plays in capturing and trafficking the viral particles. The study, published in eLife, has been developed by ICFO researchers Enric Gutiérrez, Nicolas Mateos, Kyra Borgman and Felix Campelo, led by ICREA Prof. Maria García-Parajo, in collaboration with Susana Benet from the Germans Trias i Pujol Hospital and Research Institute (IGTP), Itziar Ekizia, Núria Izquierdo-Useros and Javier Martínez-Picado from IrsiCaixa AIDS Research Institute, Jon Nieto-Garai and Maier Lorizate from the University of the Basque Country (UPV) and Carlo Manzo from the University of Vic (UVic).
Using cutting-edge techniques like super-resolution microscopy and single particle tracking, the team has been able to study the spatial organization of Siglec-1 on dendritic cell membranes and its crucial role in the early stages of infection.
Interestingly, the team found that the activation of dendritic cells leads to the formation of Siglec-1 nanoclusters, which are instrumental in enhancing the capture of HIV-like particles. Most importantly, the binding of the virus via the nanoclustering of Siglec-1 triggers a massive and global transformation of the dendritic cells’ actin cytoskeleton, which ultimately leads to the formation of a single sack-like compartment that accumulates the viruses. This virus compartment has been implicated in the spreading and infection of the T-cells by the virus leading to AIDS, but the mechanism behind its formation has been a mystery until now.
Furthermore, the researchers discovered that the organization and mobility of these nanoclusters are regulated by actin polymerization, a key cellular process that plays a role in several biological functions. They also observed that the formation of these nanoclusters and the confinement of the viruses happen in specific regions of the cell membrane characterized by RhoA activity, a protein that also plays a role in actin polymerization.
The potential of super-resolution microscopy
The use of super-resolution microscopy and single-particle tracking methods has allowed researchers to better understand the mechanisms that regulate the interaction between viruses and cells, especially the distribution and function of the receptors. “Seeing is believing!”, notes ICREA Prof. at ICFO Maria García-Parajo, “Most viruses are very small, having sizes around 100 nm, and thus not resolvable using standard optical microscopy. Even smaller are the receptors they bind to on the cell membrane. The use of super-resolution microscopy and single molecule imaging methods are therefore crucial to directly visualize how viruses are captured by cells and allow researchers to follow their fate until final infection of immune cells”.
IrsiCaixa researcher Javier Martinez-Picado also comments that “In 2012 IrsiCaixa discovered that Siglec-1 was a key protein that functions as an attachment receptor for HIV on the surface of certain immune cells, facilitating the dissemination of the virus in the body. However, the way Siglec-1 is able to capture the virus in these specific cells has remained a mystery. The current results help us to draw a more accurate picture of the HIV capture by these cells and help us develop new tools to block this mechanism”.
Although the exact role of Siglec-1 in the context of HIV-1 infection is still an area of active research, and further studies are needed to fully assess the complex interactions and its potential as a therapeutic target, these findings offer valuable insights into the complex interactions between the virus and the immune system.